Services

Gene Editing

Transgenic Services
Standard Knockout
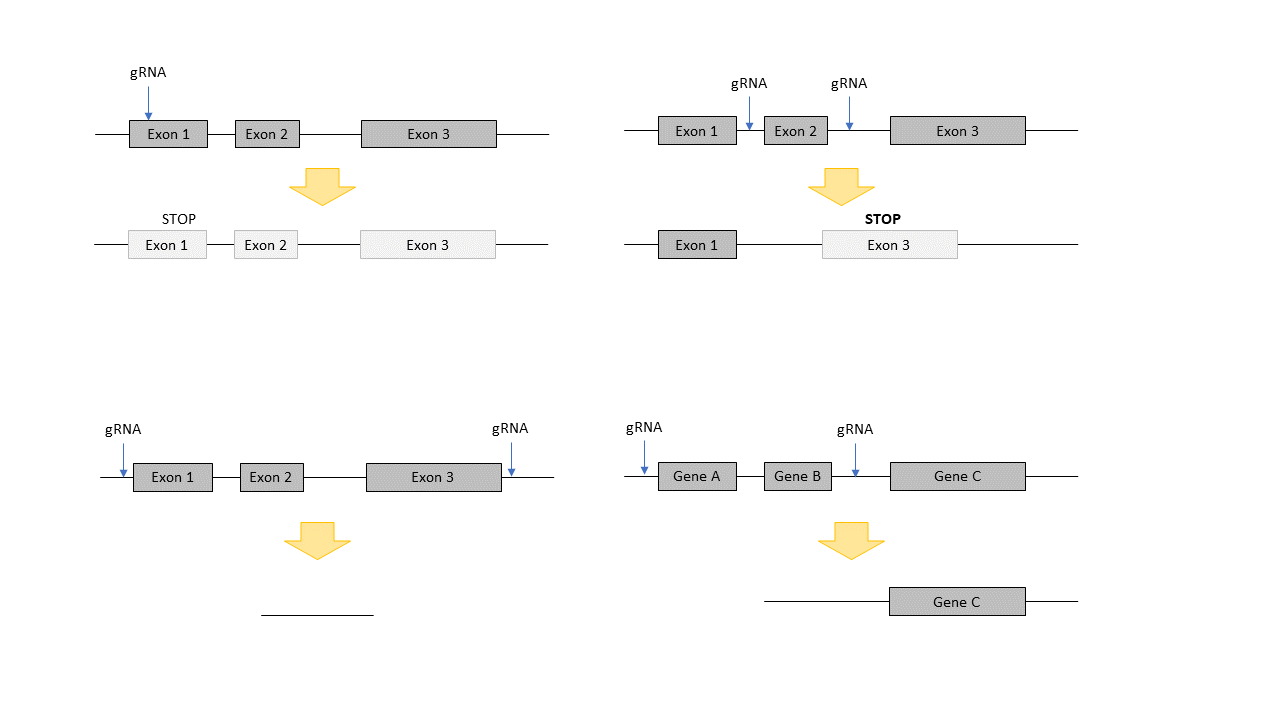
Constitutive Knockout mouse models are generated by injection or electroporation of CRISPR/Cas9 ribonucleoproteins (RNPs). These complexes consist of a small RNA, the so called guide RNA (gRNA), which determines the buiding site of the RNP complex and the Cas9 protein, which introduces the double strand break in the DNA.If you want to learn more about the priciple of CRISPR/Cas9, you find more information here.
Cutting of genomic DNA with these RNPs can lead to knockout of a gene via a frame shift induced by non-homologous end joining (NHEJ) or by cutting of crucial exons or regions in a gene, all leading to a loss of protein expression. As this mechanism is quite efficient, even deletion of larger regions or several neighboring genes are possible.
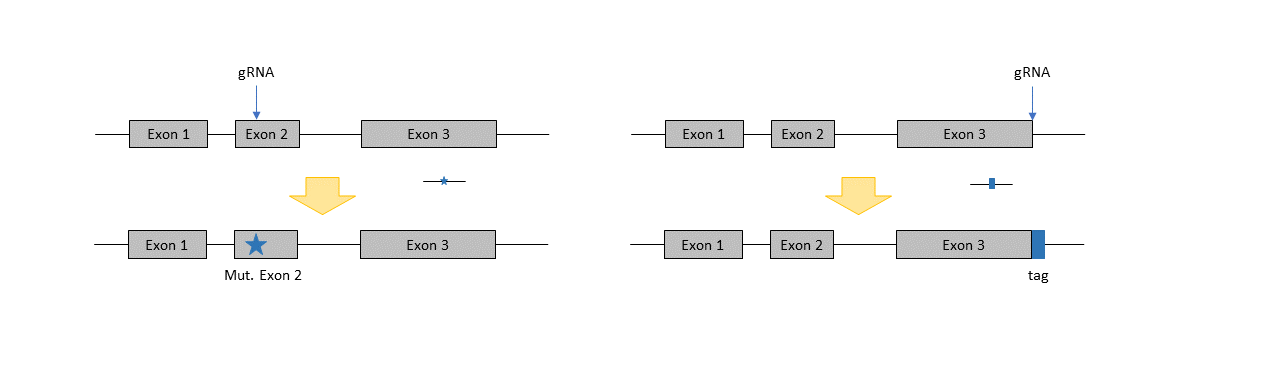
Insertions of precise mutations, small insertions and tags
When a small single-stranded oligonucleotide is added to the CRISPR/Cas9 RNPs for injection or electroporation, it is possible to insert small changes with this oligo. The cell is then applying homology directed repair (HDR) instead of NHEJ, using the oligonucleotide as repair template. HDR is slightly less likely than knockout, but still very efficient when generating small changes (up to 500 bps). Point mutations, small insertions and tags (i.e. Myc-, His-, or Flag-tags) can be inserted using this technique.
Conditional Knockout
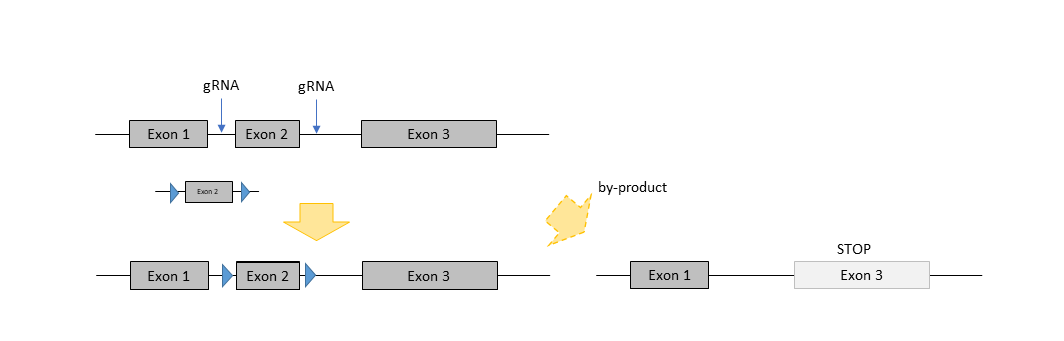
For conditional knockout of a gene, two recombination sites (like loxP-, rox- or frt-) have to be inserted flanking a crucial exon or region of a gene. This can be achieved with the technique of EASI-CRISPR (Quadros et al., 2017). With the addition of a long single-stranded oligonucleotide, HDR leads to recombination with the (twice) cut region in the genome and introduction of the recombination sites. Here, both have to be injected into the pronucleus of the oocyte. As a by-product constitutive knockout mice are often generated, too.
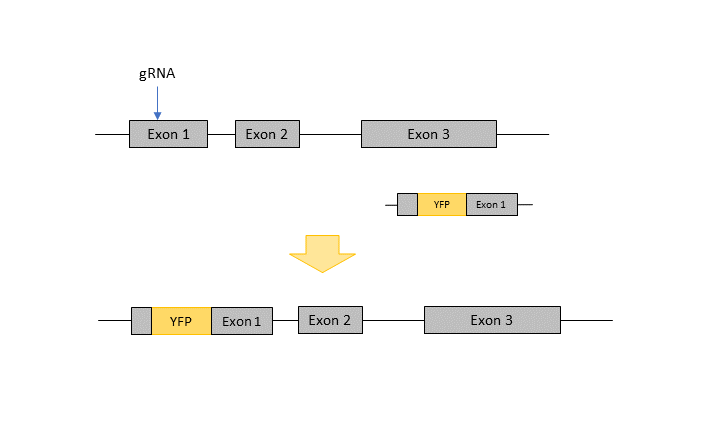
Transgene Knock-In
For the generation of larger, more complex knock-ins like fluorescent proteins or other transgenes, it depends on the gene and the insertion site whether it is possible to use CRISPR/Cas9 or “conventional” gene targeting in ES cells. For most knock-in projects we suggest the use of AAV in combination with electroporation of RNPs (CRISPR-READI, see Chen et al., 2019). Please contact us and we will discuss with you, what are the differences, the advantages and disadvantages in the usage of CRISPR/Cas9 vs. ES-cells for your project.
Blastocyst Injeciton
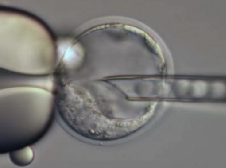
In addition to CRISPR/Cas9 based approaches we also offer „classical“ approaches for the generation of transgenic mice including blastocyst injections of modified embryonic stem cells (ES cells) as well as DNA microinjection. Please contact us for further details.
Transgenic Services
The Core Facility Gene Editing also offers standard transgenic services for import and cryopreservations of mouse lines in the animal facility of LIMES institute.

Cryopreservation

Embryo Cryopreservation
For safe cryopreservation of embryos, we freeze 300 embryos from homozygous males and 400 embryos from heterozygous males mated with wild type females. We would ask you to provide between five and ten mice, two to six months old. We will mate them with super-ovulated wild type females for which you can choose different background strains. Please inform us about any fertility issues your line might have. With this method, you conserve embryos with your complete strain-background crossed once more with the desired wild type-background.
Sperm Cryopreservation
For fast cryopreservation you can also choose to freeze sperm instead of embryos. For this, we would need at least two male mice between two and six months old, ideally proven breeders. We will freeze sperm pooled from both males in about 20 straws and test one straw for performance by in vitro fertilization (IVF) after thawing. With this method you conserve the male half of your strain-background. If desired, we can combine the IVF with an embryo transfer to directly re-derive the mouse in our SPF-facility.
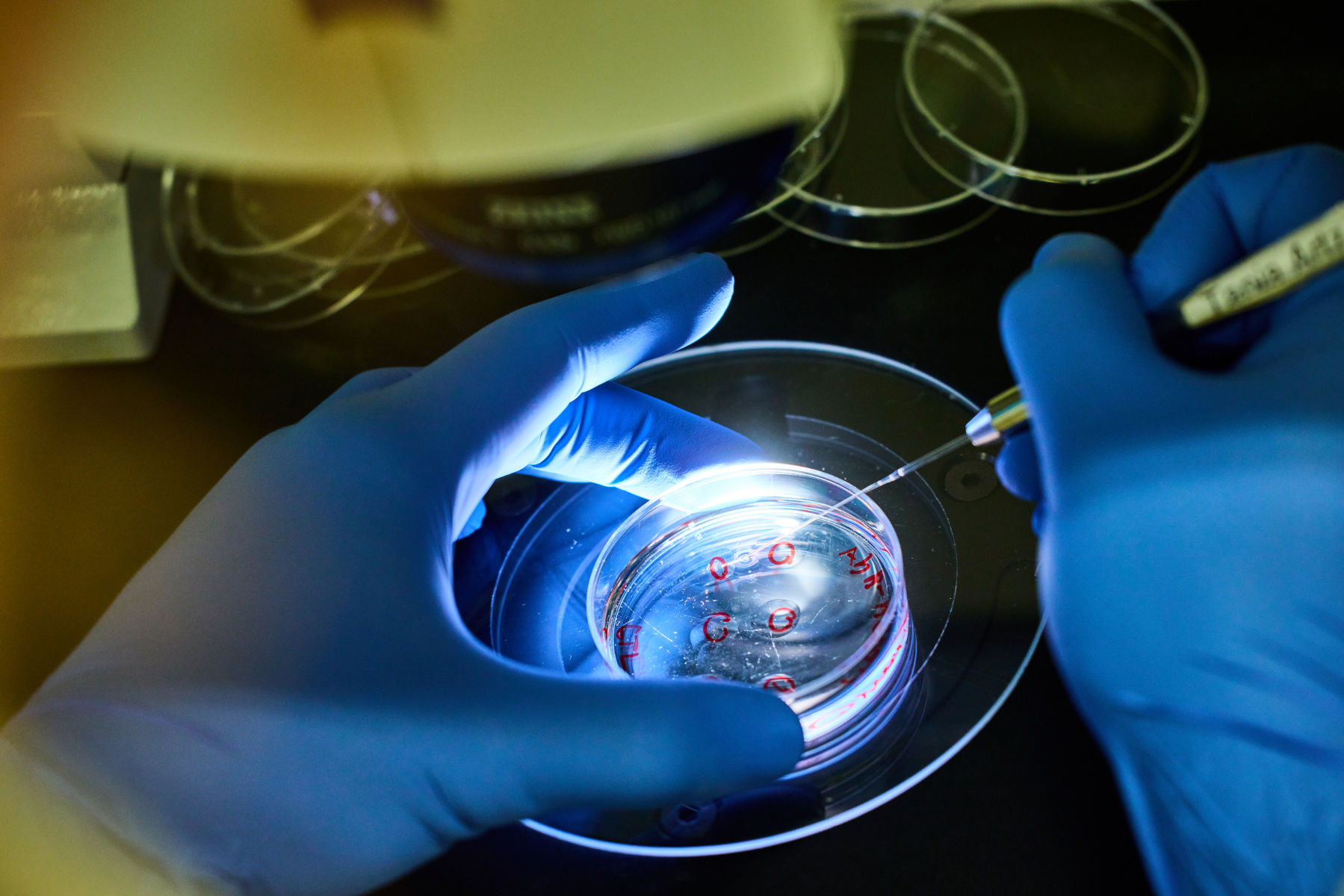
IVF and Embryo Transfer
In vitro Fertilization
To recover a mouse line from frozen sperm, we offer in vitro fertilization using donor eggs from super-ovulated wild type female mice for which you can select different mouse strains. After IVF the resulting 2-cell stage embryos are transferred into pseudo-pregnant foster mothers. After the transfer, serum from the foster mothers will be tested to make sure the mice are free of all relevant viruses (according to FELASA recommendation). A project is completed as soon as the first transgenic animal is genotyped in the clean SPF area.
Rederivation and Embryo Transfer
For rederivation of frozen embryos or establishment of imported mouse lines into our clean SPF area, we offer embryo transfer. We will mate your male mice with super-ovulated (wild type) females and transfer the resulting or revitalized embryos into foster mice. A minimum of five males or at least 50 frozen embryos would be required for this process. The import of living mice is only possible if the health status of these mice complies with our standards for the quarantine lab (for more information please send the current health certificate for your mice). If the health status does not allow import into our quarantine, import as frozen embryos or sperm (via in vitro fertilization) is possible. After the transfer, serum from the foster mothers will be tested to make sure the mice are free of all relevant viruses (according to FELASA recommendation). A transfer is completed as soon as the first transgenic animal is genotyped in the clean SPF area.
796
Embryo Transfers
since 2021
145
Strains Cryopreserved
since 2021
14
Newly generated mouse lines
since 2021
